-
 Overview
Overview
Centre Testing International Group Co., Ltd. (CTI) is a market leader in testing, inspection, certification, calibration, audit, training & technical services; building trust between governments, enterprises, and consumers.
-
 Sustainability
Sustainability
By building a full value chain ESG governance system covering the strategic decision-making level, management execution level and business operation level, it actively practices penetrating management of ESG risk and opportunities, empowering sustainable development across the industry chain.
-
 Our service
Our serviceCentre Testing International Co., Ltd. (CTI) is the pioneer and leader in the TIC Industry which provides one-stop solutions on testing, inspection, certification, calibration, audit, training & technical services.
-
By Industry
Our service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
 Environment
Environment
-
 Raw Material & Fuel Chemicals
Raw Material & Fuel Chemicals
-
 Textiles, Apparel, Footwear & Accessories
Textiles, Apparel, Footwear & Accessories
-
 Food & Agricultural Products
Food & Agricultural Products
-
 Cosmetics, Personal Care & Household Chemicals
Cosmetics, Personal Care & Household Chemicals
-
 Building Materials&Construction Engineering
Building Materials&Construction Engineering
-
 Electronic & Electrical Appliances
Electronic & Electrical Appliances
-
 Toys, Furniture & Home Decoration
Toys, Furniture & Home Decoration
-
 Industrial Equipment & Manufacturing
Industrial Equipment & Manufacturing
-
 Rail & Aviation
Rail & Aviation
-
 Automotive & Spare Parts
Automotive & Spare Parts
-
 Pharma and Medical Services
Pharma and Medical Services
-
 Maritime Vessel Compliance Testing
Maritime Vessel Compliance Testing
 By Industry
By IndustryOur service capabilties cover the upstream and downstream of the supply chain including textile and apparel,toys,electronic appliances,medical health,food...andother industries.
-
-
 Specialty
SpecialtyComprehensively guarantee quality and safety, promote compliance and innovation, demonstrate brand competitiveness, and achieve higher quality, healthier, safer, and greener sustainable development.
-
 Management
ManagementWe have established a clear governance structure in accordance with listing requirements and national regulations and policies to deal with internal and external challenges and achieve sustainable development.
-
 Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
Information DisclosureWe are committed to establishing normal and effective two-way communication with shareholders and investors. We have established a complete information disclosure mechanism to convey information to shareholders in a timely manner.
-
 Talents Policy
Talents PolicyEnsuring the basic rights and benefits of employees;
Providing professional skills training to promote employees’ growth;
Carrying out various kinds of activities to balance employees’ work and life.
-
 RecruitmentWelcome to join CTI family! We are providing a platform for you to show your talents and achieve your career aspiration.
RecruitmentWelcome to join CTI family! We are providing a platform for you to show your talents and achieve your career aspiration.

QUALITY & VALUE
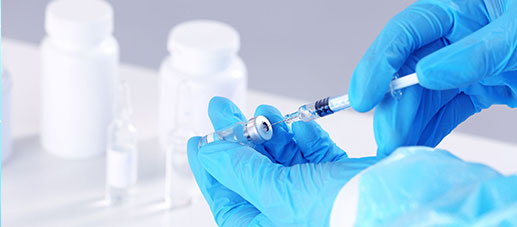
CTI Pharma and Medical Services · Drug Testing Service provides you with professional preclinical CMC services for drugs. It has an advanced pharmaceutical testing platform and an experienced experimental team. It is committed to ensuring the quality and safety of drugs; promoting the development and technological innovation of the pharmaceutical and health fields.
Service Background
Chemistry, Manufacturing, and Control (CMC) services for preclinical drugs refer to comprehensive research and services on drug synthesis and formulation, production process, quality control, etc. Before the drug enters the clinical trial stage to ensure the safety, effectiveness and quality control of the drug. These services are crucial to drug development because they provide a foundation for the clinical trials and eventual marketing of the drug.
Preclinical CMC service for drugs
CTI Pharma and Medical Service provides you with professional preclinical CMC services for drugs. It has an advanced pharmaceutical testing platform and an experienced experimental team. It is committed to ensuring the quality and safety of drugs; promoting the development and technological innovation of the pharmaceutical and health fields.
Service scope
1. Pharmaceutical Formulation: Pre-formulation research, Formulation research, Formulation reverse research (excipient determination and process route), supplementary application
2. Drug synthesis: generic drug/new drug process development, customized synthesis, synthesis process and amplification, FTE service for new molecules
3. Quality research: impurity detection method development, impurity customization and preparation, impurity conversion and removal, antibiotic polymer research
4. Registration declaration: Raw material DMF, chemical drug registration declaration
5. MAH service: apply for B certificate or drug registration approval for customers
Service items
1. Raw material CMC research service
● Impurity preparation, custom synthesis
● Generic drug registration application
● Impurity reprint and removal
● New drug IND and NDA
● Custom synthesis
● Process route development
2. Formulation CMC research service
● Eye drops, lyophilized formulation, Intralipid, liposome injections and other complex Formulation
●Development and registration application for Oral ordinary solid formulation and sustained-release formulation.
Service objects
1. Pharmaceutical manufacturers: generic drug and innovative drug companies;
2. Contract research organizations (CRO): provide impurity research and reverse phase research services for CRO companies.
3. Certified pharmaceutical sales institutions: scientific research institutions engaged in basic and applied drug research.
Technology platform and research
1. Instruments and equipment
Thermo Fisher Scientific Orbitrap Fusion Lumos
Thermo Fisher Scientific Orbitrap Q Exactive HF
2D-HPLC-Q-tof
AB SCIEX LC-MS/MS 7500
AB SCIEX LC-MS/MS 5500+
Agilent GC-MS 8890-5977B
Thermo iCAP-RQ ICP-MS
2.Technology research
two-dimensional liquid chromatography-mass for impurity research;
Deuterated drug synthesis
Carbohydrate microchip technology platform
Our advantages
? Raw material registration and declaration, high-difficulty drug synthesis, deuterated drug synthesis;
? Drug quality research, especially impurity research and complex development
? Injections and Intralipid of complex injections
? Development of freeze-dried preparations and development of sustained-release preparations
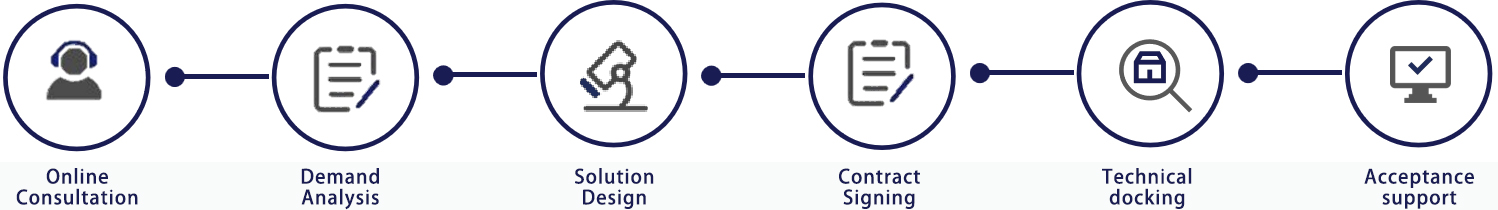
Service process























 粵公網(wǎng)安備 44030602000441號(hào)
粵公網(wǎng)安備 44030602000441號(hào)